Section One: Introduction
The administrative system for Traditional Chinese Medicine Management changed several times as the government system was reformed from the first year of the Republic of China (1911) until now. The TCM Committee was set up under the Department of Health within the Ministry of the Interior after the government relocated to Taiwan. This committee provided advice and recommendations to health authorities regarding the development of TCM. The Department of Health was transferred from under the Ministry of Interior to the Executive Yuan in 1971. The TCM Committee has remained since its establishment by the Executive Yuan. TCM management services grew rapidly due to strong worldwide demand for TCM. The Committee of Chinese Medicine and Pharmacy was officially established by the Executive Yuan on November 1, 1995. This institution is not only an administrative service, but is also a critical point in TCM development history. The Committee includes four services teams: Chinese medicine, Chinese pharmacy, research and development, information resources and records. It is also responsible for TCM administration and services promotion.
Owing to the reorganization of the Executive Yuan, the Ministry of Health and Welfare (MOHW) was established on July 23, 2013 to combine the former original Department of Health of the Executive Yuan with the social service administration responsibilities of the Ministry of the Interior. The TCM Committee of the Health Department in the Executive Yuan was incorporated into the Ministry of Health and Welfare and re-named as the Department of Chinese Medicine and Pharmacy (DCMP). The position was upgraded from a level three to a level two institution. "The National Research Institute of Chinese Medicine", formerly under the Department of Education, was renamed "National Research Institute of Chinese Medicine, Ministry of Health and Welfare" as a level three institution. The Research Institution co-operates with the DCMP to promote the development of TCM in Taiwan.
Section Two: Organization of the Ministry of Health and Welfare (MOHW)
The Ministry of Health and Welfare was newly established in 2013. It included the former Executive Yuan's Department of Health and five sub-institutions: the Department of Social Affairs from the Ministry of Interior, the Children Bureau, the Committee for Prevention of Domestic Violence and Sexual Assault, the National Pension Supervisory Board, and the National Research Institution of TCM from the Department of Education. After reorganizing, the MOHW became a Ministry with 9 departments, 6 divisions, 6 level-three agencies (institutions), 26 ministerial hospitals, and 13 social welfare institutions (figure 1-1). The Department of Chinese Medicine and Pharmacy, the Department of Medical Affairs which administers Western medicine, and the Department of Mental and Oral Health which administers dentistry are among the 9 MOHW departments.
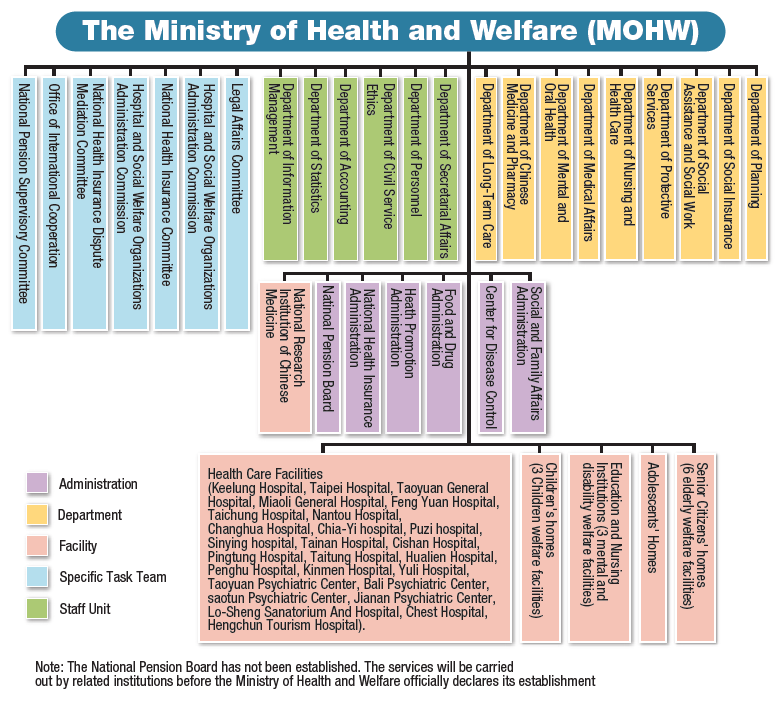
Figure 1-1: MOHW organization
Section Three: The Duty of the Department of Chinese Medicine and Pharmacy in MOHW
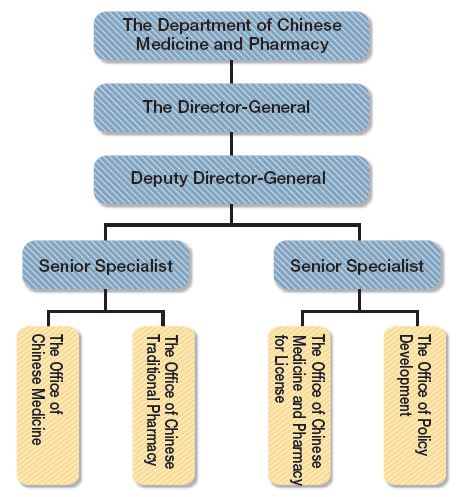
I.The Organizational Structure
Figure 1-2: Organization Structure for the Department of Chinese Medicine and Pharmacy
II.Functions of the Department of Chinese Medicine in the Ministry of Health and Welfare:
- Planning and promotion of administrative policies for TCM and related regulations.
- Planning and promotion of policies for medical human resource growth, the administration of TCM workers, and related regulations.
- Planning and promotion of administrative policies for TCM health care facilities and related regulations.
- Planning and promotion of administrative policies for TCM and herbs, quality improvement and related regulations.
- Other items regarding Traditional Chinese Medicine administration.
Section Four:The Formulation of Chinese Medicine and Pharmacy Development Act
I.The Promotion Course
The World Health Organization's (WHO) Traditional Medicine Strategy 2014-2023 calls for nations to place emphasis on traditional medicine and develop management legislatures, as well as construct a robust regulations system and promote traditional medicine's contribution to health for all. Paragraph 5 of Article 10 of the Additional Articles of the Constitution of the R.O.C. (Taiwan) also stipulated that: "The State shall promote universal health insurance and promote the research and development of both modern and traditional medicines." To fulfill the duties entrusted to it by the Constitution, and to draft unique legislation that both encapsulates Taiwan's TCM characteristics and keeps in line with international trends, the Ministry has endeavored since 2017 to carry out pre-planning and research for the legislation of the Chinese Medicine and Pharmacy Development Act. The act was submitted for review by the Executive Yuan on October 18, 2019 and was approved during the 3,676th Executive Yuan Sittings on November 14 of the same year. The act was then submitted for review by the Legislative Yuan, and through the concerted efforts of majority and minority political parties, the act was approved on December 6 of the same year and promulgated by the President on December 31 (Figure 1-3).
The enactment of this legislation symbolized a new milestone in the development history of Taiwan's Traditional Chinese Medicine and laid the principal foundation for the nation's TCM development, as well as establishing the basis of administrative and financial support. It is hoped that the formulation of this act will enhance the sustainable development of TCM and pharmacy for the purpose of improving the health and well-being of all. Measures to achieve these goals involve: planning and promotion of TCM development projects, implementation of TCM healthcare services, promotion of Chinese pharmaceutical industry quality and development, co-existence of traditional knowledge and innovative scientific development, enhancement of TCM research and talent development, and the sharing of Taiwan's TCM experiences with the international community.
Figure 1-3: Presidential Office Gazette of Chinese Medicine and Pharmacy Development Act
II.The Focus of the Chinese Medicine and Pharmacy Development Act
The Act is comprised of 7 Chapters and 24 Articles. The key principles of the act are as follows:
- Promoting sustainable development of Chinese Medicine and Pharmacy policies. The act calls for a central competent authority to formulate development plans for Chinese medicine and pharmacy every five years in order to secure the necessary financial and administrative resources; the establishment of research funds for TCM and searching for new financial resources to maintain TCM research capacity and transform results of evidence-based research into practical applications; promotion of TCM R&D and provision of appropriate incentives or subsidies to promote innovative development of Chinese pharmaceutics and cultivation of medicinal plants.
- Perfecting of TCM healthcare services: enhance the roles and functions of Chinese Medicine and pharmacy in universal healthcare; improve the accessibility and quality of Chinese Medicine medical care; develop preventive healthcare and household care using a TCM specialty; promote collaboration between Western medicine and Chinese Medicine; engage in diversified Chinese Medicine healthcare services; promote the utilization and development of Chinese Medicine healthcare services.
- Enhancing the quality management of TCM industry development: develop the cultivation of medicinal plants in Taiwan, provide incentives and guarantee of land lease; strengthen monitoring procedures for marketed Chinese pharmaceutics and publicly announce the results; perfect management regulations to improve the quality of Chinese pharmaceutics.
- Advancing TCM research and development and talent cultivation: construct a national TCM knowledge database; develop evidence-based TCM, promote innovation and TCM R&D; enhance international exchange; preserve traditional crafts and knowledge of TCM to allow co-existence and development of traditional and modern medical knowledge; perfect TCM workforce planning, cultivate talents in TCM-related technologies, and popularize TCM knowledge and education for the public.
To promote the sustainable development of TCM, the Ministry is planning a prospective TCM development blueprint in line with the fundamental vision of the Chinese Medicine and Pharmacy Development Act (Figures 1-4). The aim is to perfect TCM healthcare, strengthen TCM industry and quality management, promote research development and talent training, and showcase the unique features of Taiwan's TCM, while exerting influence in the international community for the purpose of health and well-being for all.
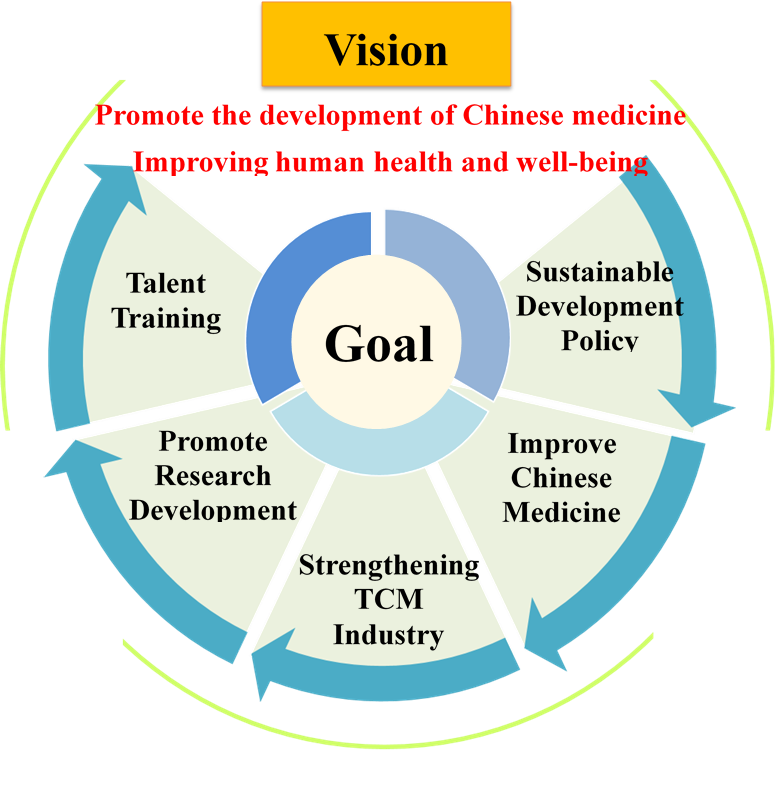
Figure 1-4: The Vision of the Chinese Medicine and Pharmacy Development Act
III.The Effects of the Chinese Medicine and Pharmacy Development Act
By promulgating and gradually implementing the Chinese Medicine and Pharmacy Act, it is expected that the management of Chinese Medicine and Pharmacy will further perfect the management of Chinese Medicine and Pharmacy. The implications are two-fold: in terms of Chinese Medicine, a training system for Specialist Chinese Medicine physicians will be established in the future; precision Chinese Medicine and smart Chinese Medicine healthcare models are under development, as well as Chinese Medicine community and household healthcare networks. A specialist Chinese Medicine household medical care team comprising Chinese Medicine institutions, local Chinese Medicine associations, pharmacists and nursing staff is also expected in the future to provide medical and long-term care services.
In terms of Chinese pharmacy, we plan to encourage the cultivation of TCM medicinal plants in Taiwan. Medicinal plants like red sage root and rhizome, turmeric rhizome and Bletilla formosana (Hayata) Schltr. tuber are already being cultivated in Taiwan. As the harvesting periods of medicinal plants are generally longer, land-leasing guarantees and protection will be provided for the cultivation of TCM medicinal plants on publicly-owned lands, which will reduce the costs and pressures related to contract amendment and land recovery. Additionally, relevant sub-regulations will also be formulated to provide appropriate incentives or subsidies for plant farmers, which will cut down dependence on imported TCM ingredients.
To strengthen management of the quality control of TCM, we referenced domestic and international development trends to revise the Taiwan Herbal Pharmacopeia, amending the limit standards for abnormal substances in TCM ingredients (such as sulfur dioxide, heavy metals, aflatoxins and pesticide residues). For TCM concentrated preparations, we will gradually devise standards for the concentration of marker component, improving quality control of TCM products. In the future, we also plan to devise related regulations to enhance the monitoring of marketed TCM preparation and ingredient, in order to safeguard the use of TCM for the general public.
As the competition for global traditional pharmaceutics becomes fiercer in recent years, we seek to stimulate the development of the TCM industry and establish a comprehensive quality management system for both TCM products and TCM manufacturing environments. The Ministry is also pro-actively promoting the globalization of quality management standards for TCM pharmaceutical factories. Starting from January 1, 2020, TCM pharmaceutical factories will undergo validation in four stages, which will gain the industry increased international recognition.